physical organic chemistry
Andrzej Sygula

Synthesis of Buckybowls: Curved-Surface Polycyclic Aromatic Hydrocarbons (PAHs)
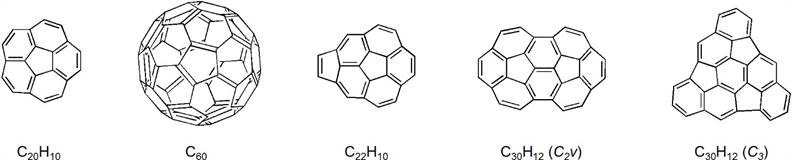
Corannulene (C20H10), the smallest buckybowl was first synthesized in 1966 (the “pre-fullerene” era) by Bath and Lawton, represents the polar cap of buckminsterfullerene C60. The original synthesis comprised of 17 laborious steps with an overall yield of 0.4%. The novelty of the bowl-shaped PAH attracted considerable attention, but the studies of its chemistry and properties were scarce because of the very limited supply of the compound. In 1990s introduction of the flash vacuum pyrolysis (FVP) methodology provided a more attractive alternative to produce corannulene on a milligram scale. Larger buckybowls were also prepared by FVP and our research group contributed to the field synthesizing cyclopentacorannulene (C22H10), with the additional five-membered ring freezing the fast bowl-to-bowl inversion of the corannulene system, and two isomeric semibuckminsterfullerenes C30H12, representing one-half of buckminsterfullerene surface.
Corannulene (C20H10), the smallest buckybowl was first synthesized in 1966 (the “pre-fullerene” era) by Bath and Lawton, represents the polar cap of buckminsterfullerene C60. The original synthesis comprised of 17 laborious steps with an overall yield of 0.4%. The novelty of the bowl-shaped PAH attracted considerable attention, but the studies of its chemistry and properties were scarce because of the very limited supply of the compound. In 1990s introduction of the flash vacuum pyrolysis (FVP) methodology provided a more attractive alternative to produce corannulene on a milligram scale. Larger buckybowls were also prepared by FVP and our research group contributed to the field synthesizing cyclopentacorannulene (C22H10), with the additional five-membered ring freezing the fast bowl-to-bowl inversion of the corannulene system, and two isomeric semibuckminsterfullerenes C30H12, representing one-half of buckminsterfullerene surface.
Despite the undisputable success of the FVP methodology a need for practical, solution-based large scale synthesis of buckybowls has become evident if these novel systems were to be thoroughly studied. Our DOE sponsored program achieved a major breakthrough with a development of a solution, multigram scale synthesis of the corannulene framework with excellent yields (>80%) by simply refluxing 1,6,7,10-tetrakis(dibromomethyl)fluoranthene in aqueous dioxane with a little sodium hydroxide.
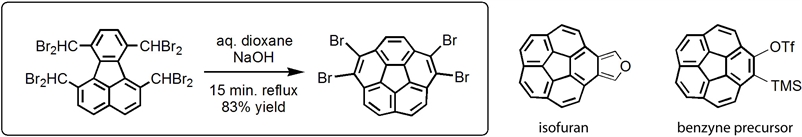
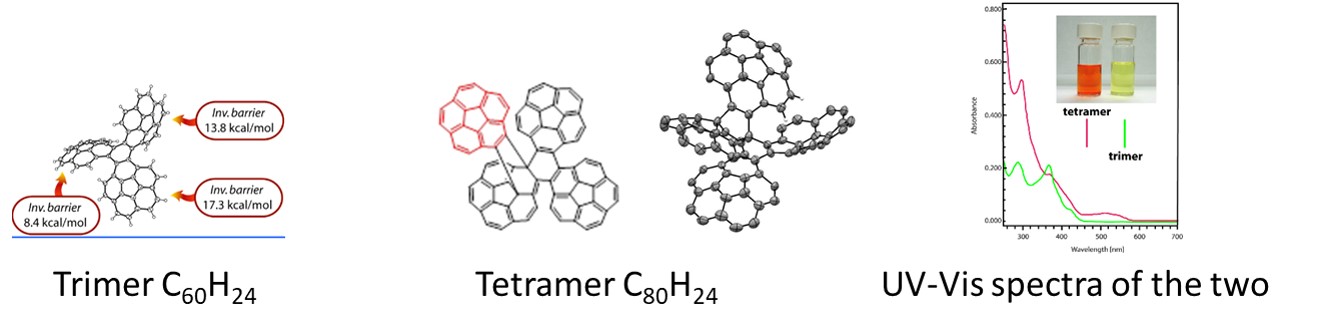
Consequently, we have introduced two corannulene-based synthons, i.e. isocorannulenefuran, a reactive diene, and o-TMS triflate, a precursor for 1,2-didehydrocorannulene (benzyne), a reactive dienofile. Several large PAHs with corannulene subunits were prepared by the Diels-Alder cycloaddition of the synthons with proper dienofiles or dienes. As an example, Palladium catalized cyclotrimerization of corannulyne generated from o-TMSl triflate precursor provided a highly distorted timer C60H24 exhibiting an interesting conformational behaviour driven by dramatically different bowl-to-bowl inversion barriers of formally identical corannulene subunits. The calculated bowl-to-bowl inversion barriers are in the range of 8.4 to 17.3 kcal/mol, apparently tuned by the steric hindrance. Distortion of the corannulene subunits in the cyclotrimer resulted in its unprecedented Diels-Alder reaction with another corannulyne unit resulting in the formation of tetrameric C80H32 hydrocarbon, the largest oligomer of corannulyne reported and fully characterized to date. Its conformational behavior and photophysical properties are dramatically different as compared to the trimer.
Molecular Clips and Tweezers with Corannulene Pincers: Buckycatchers
The accessible concave surfaces of buckybowls make them the potential candidates for the efficient molecular receptors for fullerenes with the ability to recognize curved-surfaces of the carbon cages through the concave-convex “ball-and-socket” pie-pie interactions. Despite a substantial gas-phase binding energy predicted for 1:1 assembly of corannulene with C60, the supramolecule has not been detected in solution, presumably due to the significant solvation and/or entropy penalties. However, efficient molecular receptors for fullerenes can be prepared if two or more buckybowl pincers are prearranged on a proper tether. We have concentrated on the design and synthesis of such receptors and in 2007, we prepared Buckycatcher 1 with two corannulene pincers and a tetrabenzocyclooctatetraene tether. The molecular clip forms stable inclusion complexes with C60 both in solution and in the solid state.
The accessible concave surfaces of buckybowls make them the potential candidates for the efficient molecular receptors for fullerenes with the ability to recognize curved-surfaces of the carbon cages through the concave-convex “ball-and-socket” pie-pie interactions. Despite a substantial gas-phase binding energy predicted for 1:1 assembly of corannulene with C60, the supramolecule has not been detected in solution, presumably due to the significant solvation and/or entropy penalties. However, efficient molecular receptors for fullerenes can be prepared if two or more buckybowl pincers are prearranged on a proper tether. We have concentrated on the design and synthesis of such receptors and in 2007, we prepared Buckycatcher 1 with two corannulene pincers and a tetrabenzocyclooctatetraene tether. The molecular clip forms stable inclusion complexes with C60 both in solution and in the solid state.
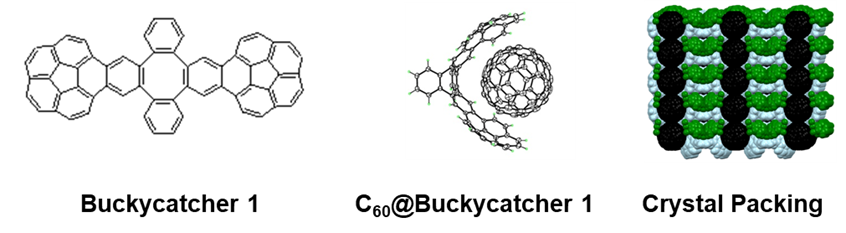
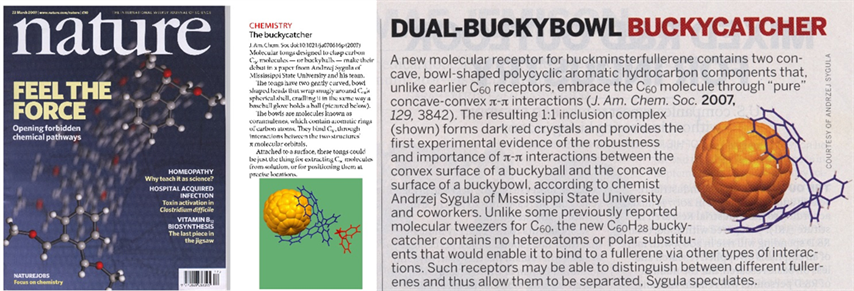
A single-crystal X-ray crystallography of the complex showed that the fullerene molecule is placed nicely in the center of a doubly concave cleft of Buckycatcher with most of the corannulene pincers carbon atoms being in van der Waals contact with the fullerene cage. These results provided the first experimental evidence for the importance of concave-convex pie-pie interactions in the supramolecular chemistry of fullerene carbon cages and buckybowls. The discovery, published in J. Am. Chem. Soc. 2007, 129, 3842, was announced in the Research Highlights sections of Nature (2007, 446, 360), Chem. Eng. News (2007, 85, 37) and Nature Nanotech. (2007, March 23). To date, the original paper has been cited over 350 times.
Engineering the Tethers by "Intelligent Design"
We have switched from the usual heuristic approach (which involves synthesis of randomly selected potential clips followed by the testing of their binding abilities), to the “intelligent design” approach, in which the guest-binding potential of the receptor could be a priori estimated to some degree. While the practical computational models of solvation are being developed they are still not accurate enough to be used for the “intelligent design” of our receptors. On the other hand, a quick a priori assessment of the binding potential of a receptor is possible if one compares the gas-phase binding energies of a series of structurally similar molecular clips. It is then assumed that the entropy and solvation penalties are comparable within the series and that the binding energy governed by the pincers’ topology becomes the decisive factor determining the receptor’s affinity toward a given guest. Applying this simple approach to a series of bis-corannulene clips we have selected and prepared two molecular receptors with a higher affinity toward fullerenes than Buckycatcher 1. The first, C51H24, dubbed Buckycatcher 2, (Angew. Chem. Int. Ed. 2015, 54, 11153) comprises two corannulene pincers on a dibenzobornadiene tether. As demonstrated by NMR titration, Buckycatcher 2 exhibits an order of magnitude higher association constants with fullerenes than Buckycatcher 1.
We have switched from the usual heuristic approach (which involves synthesis of randomly selected potential clips followed by the testing of their binding abilities), to the “intelligent design” approach, in which the guest-binding potential of the receptor could be a priori estimated to some degree. While the practical computational models of solvation are being developed they are still not accurate enough to be used for the “intelligent design” of our receptors. On the other hand, a quick a priori assessment of the binding potential of a receptor is possible if one compares the gas-phase binding energies of a series of structurally similar molecular clips. It is then assumed that the entropy and solvation penalties are comparable within the series and that the binding energy governed by the pincers’ topology becomes the decisive factor determining the receptor’s affinity toward a given guest. Applying this simple approach to a series of bis-corannulene clips we have selected and prepared two molecular receptors with a higher affinity toward fullerenes than Buckycatcher 1. The first, C51H24, dubbed Buckycatcher 2, (Angew. Chem. Int. Ed. 2015, 54, 11153) comprises two corannulene pincers on a dibenzobornadiene tether. As demonstrated by NMR titration, Buckycatcher 2 exhibits an order of magnitude higher association constants with fullerenes than Buckycatcher 1.
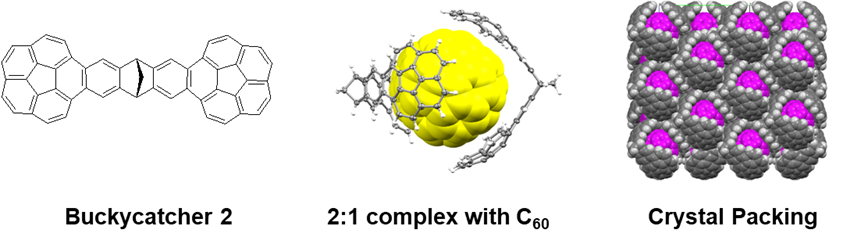
In addition to the usual 1:1 inclusion complexes, 2:1 complexes of Buckycatcher 2 with both C60 and C70 were detected in solution and in the solid state. The fullerene cage is accommodated by two bis-corannulene receptors in a nanometric-size universal joint arrangement. Indeed, computational screening indicates the conformational behavior expected for a universal joint.
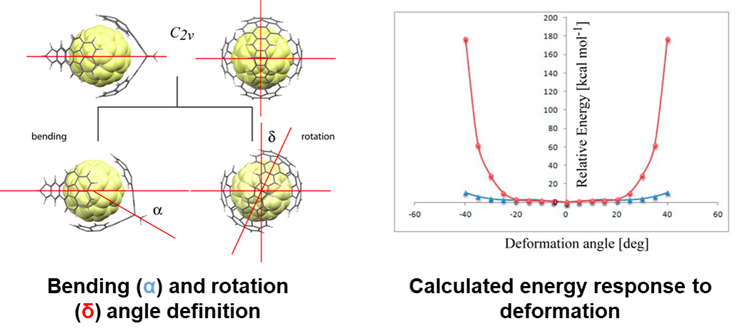
Bending of the assembly up to alpha ~ 40 deg (i.e. rotating of one of the hinges in its Cs plane of symmetry while keeping the remaining two components frozen; blue line above) is a low energy process indicating a high degree of flexibility. On the other hand, rotation of one of the hinges along the C2 symmetry axis defined by the two apical norbornadiene carbon atoms significantly increases the total energy of the assembly for the rotation angles gama exceeding 20 deg (red line above) due to the steric hindrance of the rim hydrogen atoms of the clips. The hindrance would potentially allow for a transmission of the rotary motion from one of the “hinges” to the other.
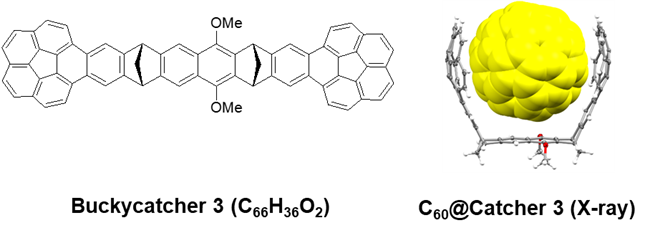
Buckycatcher 3: Reaching the Affinity Limits?
As predicted by our computational study, bis-corannulene receptors preorganized in a syn-fasion on one of the Klärner’s tethers exhibits an exceptional affinity toward fullerene cages. Both C60 and C70 are strongly bound by Buckycatcher 3 in all solvents tested (Org. Lett., 2015, 17, 5292). Like in the case of Buckycatcher 2, both 1:1 and 2:1 associates with fullerenes are present in solutions. Buckycatcher 3 exceeds the performance of the former receptors by ca. 2 orders of magnitude and, in addition, shows an enhanced preference for C70 over C60. As demonstrated by X-ray crystal structure determination, the tether in Buckycatcher 3 not only preorganizes the corannulene pincers ii nearly perfect topology of the host, but also contributes to the dispersion-based binding of the fullerene cage.
As predicted by our computational study, bis-corannulene receptors preorganized in a syn-fasion on one of the Klärner’s tethers exhibits an exceptional affinity toward fullerene cages. Both C60 and C70 are strongly bound by Buckycatcher 3 in all solvents tested (Org. Lett., 2015, 17, 5292). Like in the case of Buckycatcher 2, both 1:1 and 2:1 associates with fullerenes are present in solutions. Buckycatcher 3 exceeds the performance of the former receptors by ca. 2 orders of magnitude and, in addition, shows an enhanced preference for C70 over C60. As demonstrated by X-ray crystal structure determination, the tether in Buckycatcher 3 not only preorganizes the corannulene pincers ii nearly perfect topology of the host, but also contributes to the dispersion-based binding of the fullerene cage.